Pathophysiology
Pathophysiology of Basal Cell Carcinoma
The Sonic Hedgehog (SHH) pathway is important for the organogenesis of almost all organs in mammals, as well as in regeneration and homeostasis. Further, SHH signaling is disrupted in diverse types of cancer. Aberrant signaling of the SHH pathway is important to the pathophysiology of BCC, being a principal driver in progression and pathogenesis of the disease.
Figure 1. Sonic Hedgehog (SHH) Pathway
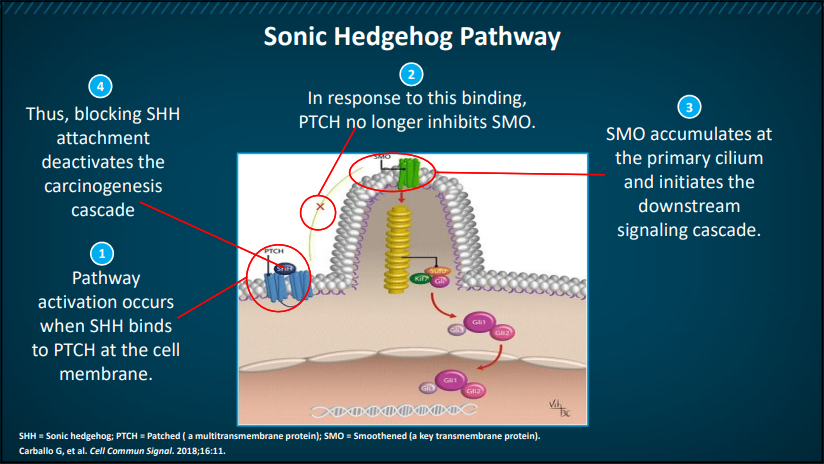
As depicted above in Figure 1, SHH pathway activation occurs when SHH binds to Patched (PTCH) on the cell membrane. As a result, Smoothened (SMO), a key transmembrane protein, is no longer inhibited, leading to its accumulation at the primary cilium. SMO accumulates and initiates the downstream signaling cascade, which results in the translocation of proteins to the nucleus and resulting transcription of target genes, including Ptch1 and Gli1. Blocking SHH helps to deactivate this carcinogenic cascade.
Immune Dysfunction and Tumorigenesis
With ultra-violet light exposure as the dominant risk factor for BCC, these tumors harbor high mutational burdens. Highly mutated tumors are more likely to express immunogenic tumor neoantigens that attract effector T-cells, which can be unleashed by blockade of the PD-1 immune checkpoint.
The development of cancer immunity is a cyclic process (Figure 2) that can be self-propagating, leading to an accumulation of immune-stimulatory factors that in principle should amplify and broaden T-cell responses. The cycle is also characterized by inhibitory factors that lead to immune regulatory feedback mechanisms, which can halt the development or limit the immunity. This cycle can be divided into seven major steps, starting with the release of antigens from the cancer cell and ending with the killing of cancer cells. In Figure 2, the individual steps are described, with the primary cell types involved and the anatomic location of the activity.
Fig 2. Cancer Immunity Cycle
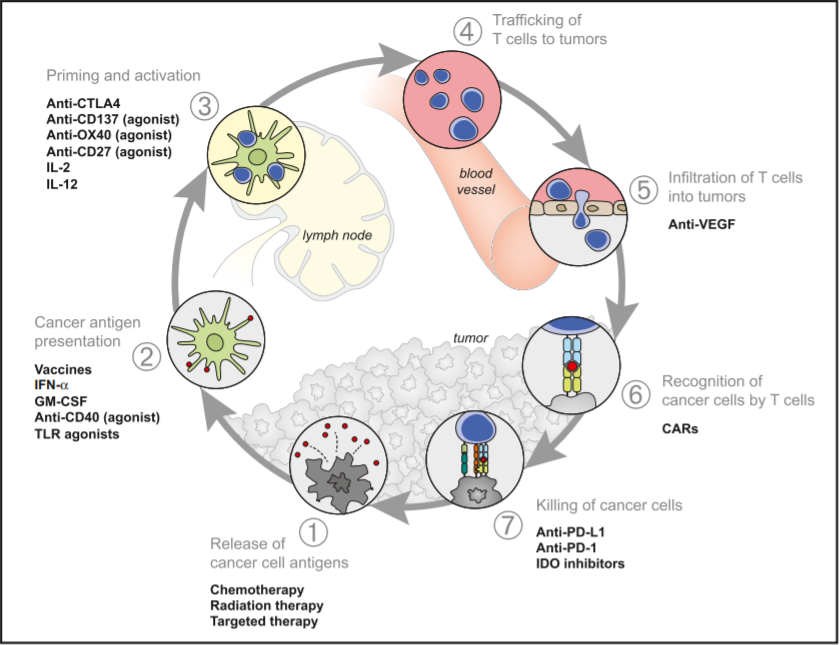
In short, the cancer antigens (1) are presented by dendritic cells (DCs) to T cells (2) resulting in priming/activation (3) of effector T cells with T cell responses against the cancer-specific antigens that are recognized as foreign. The activated effector T cells travel to (4) and infiltrate (5) the tumor where they specifically recognize and bind to cancer cells through the interaction between their T cell receptor (TCR) and its antigen bound to MHCI (6). The cytotoxic T lymphocytes (CTLs) then kill their target cancer cell (7), releasing more antigen resulting in an amplification and broadening of the immune response.
In patients with cancer, this immunity cycle is often dysfunctional in a variety of ways:
- Tumor antigens may not be detected.
- DCs and T cells may treat antigens as self rather than foreign thereby creating T regulatory cell responses rather than effector responses.
- T cells may not properly seek out tumors or may be inhibited from infiltrating the tumor.
- Factors in the tumor microenvironment might suppress effector cells that are produced.
