Systemic Treatment
Systemic Treatment of Advanced BCC
Recent systemic treatment for patients with advanced or metastatic BCC that is untreatable with conventional therapeutic methods, including Hedgehog pathway inhibitors and immunotherapy, have become cornerstones for advanced and metastatic cutaneous tumor management.
In specific populations, such as the immune-suppressed, the elderly, those with poor baseline functional status, and cases of metastatic, advanced, recalcitrant, or cosmetically sensitive disease, nonsurgical management may be a desirable alternative.
Aberrant Metabolic Pathways
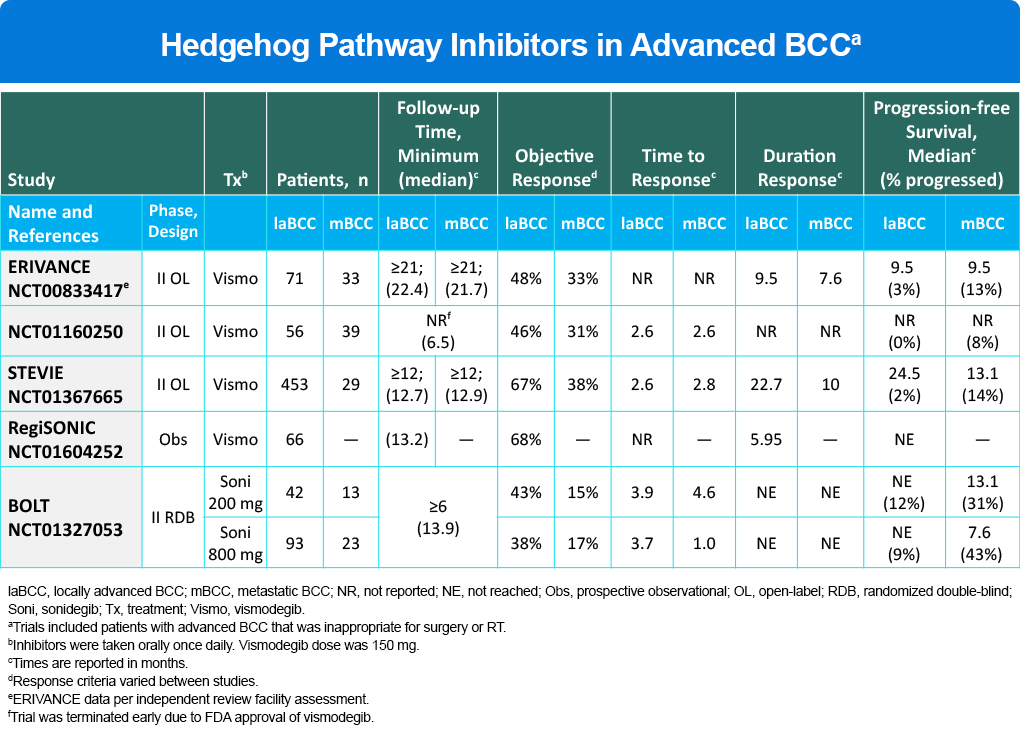
Aberrant signaling of the hedgehog pathway is important to the pathophysiology of BCC, as it is a principal driver in progression and pathogenesis of the disease. As a key therapeutic target, two hedgehog pathway inhibitors, vismodegib and sonidegib, are currently indicated for the treatment of locally advanced (laBCC) in cases where surgery and radiotherapy are inappropriate. Based on 21 month minimum follow-up, objective response to vismodegib was 48% (laBCC) and 33% (metastatic) (Table 1). A second inhibitor, sonidegib, was also approved based on BOLT study data with an ORR of 43% (laBCC) and 15% (metastatic) for the lower dose (Table 1). A key limitation to these therapies is development of resistance which limits response (Table 1). In studies, both vismodegib and sonidegib demonstrated serious AEs leading to discontinuation in 14% – 32% of patients. The most commonly reported adverse effects included nausea, dysgeusia, muscle spasm, alopecia, weight loss, diarrhea, and fatigue. The NCCN guidance in the management of laBCC or metastatic disease, states, “Due to the frequency of intolerable side effects associated with hedgehog pathway inhibitors, drug holidays or other alternatives to daily dosing can be used to reduce side effects to improve adherence to therapy and quality of life.”
Table 1. Hedgehog Pathway Inhibitors in Advanced BCC
Initiating Anticancer Immunity: Antigen Release, Presentation, and T Cell Priming
Attempts to activate or introduce cancer antigen-specific T cells, as well as stimulate the proliferation of these cells over the last 20 years have led to mostly no, minimal or modest appreciable anticancer immune responses. For many human cancers, the cancer-immunity cycle is intact up to the point of tumor cell killing by T-cells, which can be potently restrained by PD-L1. Once the PD-L1/PD-1 interaction is blocked, preexisting anticancer T-cells can have their effector function rapidly restored.
To leverage this understanding of both the SHH pathway and the immune system in the tumorigenesis of BCC, a small study of 16 patients were enrolled (9 on pembrolizumab alone, 7 on pembrolizumab/vismodegib). The ORR for all patients was 38% (44% for the pembrolizumab and 29% for dual-therapy), and there were three with CRs (one with pembrolizumab monotherapy, two with dual therapy). There was no sign of unexpected toxicity with the combination, but no clear signal that combining the two agents resulted in improved efficacy.
Cemiplimab, a monoclonal antibody to PD-1, is the first and currently the only immunotherapy approved by the FDA for BCC. It has demonstrated efficacy with a safety profile comparable with that of other anti–PD-1 agents in advanced malignancies. Data looking at 112 patients with BCC (84 locally advanced, 28 metastatic) resulted in accelerated FDA approval. Among the 84 patients with laBCC, the confirmed ORR was 29%, with 79% of responders maintaining their response for at least 6 months. Among the 28 with mBCC, the confirmed ORR was 21%, and all responders maintained their responses for at least 6 months. Severe adverse reactions were immune-mediated (eg, pneumonitis, hepatitis, colitis, adrenal insufficiency, hypo- and hyperthyroidism, diabetes mellitus and nephritis) and infusion reactions. The most common adverse reactions (incidence ≥20%) were fatigue, musculoskeletal pain, diarrhea, rash, and pruritus.
Figure 3. Therapies That Affect the Immune Cycle
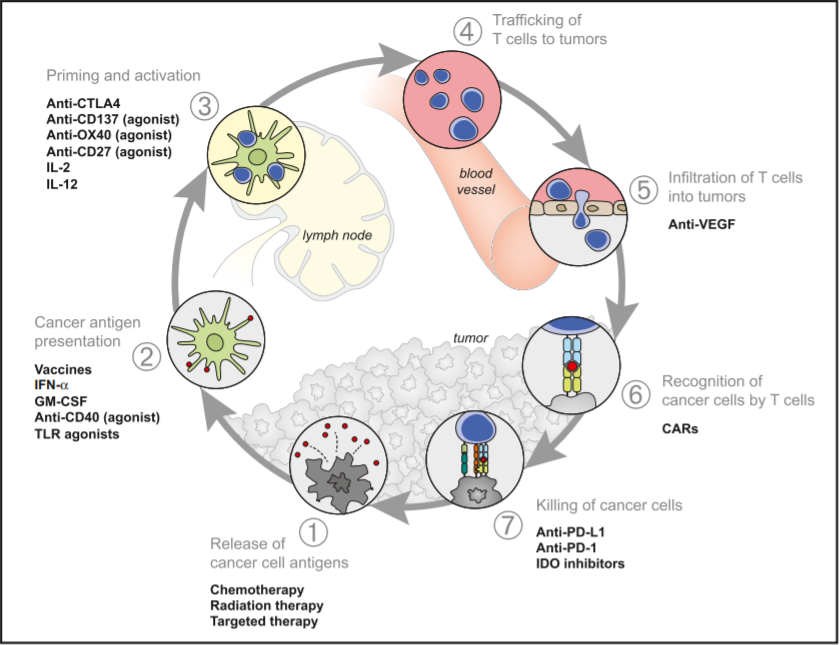
Chemotherapy and radiation therapy have long histories in the treatment of BCC with their release of neoantigens (Figure 3) being a well-recognized trigger for the individual’s immune system. More recently the leveraging of immune anti-tumorigenic effects have been with successful with the clinical application of both CTLA-4 and PD-1/L1 inhibitors across multiple cutaneous cancers. CTLA-4 inhibition is intimately involved with the priming and activation of T cells (step 3) while PD-1 and PD-L1 inhibitors effects are seen further down the immune cycle (step 7), involving the re-vitalizing of T cells so they effectively recognize and kill cancer cells.
Immune checkpoint inhibitors are an exciting new class of therapeutics that may soon provide clinicians and patients with a number of treatment options that far outstrip the use of chemotherapy.
References
Bassett-Seguin N, Hauschild A, Grob JJ, et al. Vismodegib in patients with advanced basal cell carcinoma (STEVIE): A pre-planned interim analysis of an international, open-label trial. Lancet Oncol. 2015;16:729-736.
Chang AL, Solomon JA, Hainsworth JD, et al. Expanded access study of patients with advanced basal cell carcinoma treated with the Hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2014;70:60-69.
Chang ALS, Tran DC, Cannon JGD, et al. Pembrolizumab for advanced basal cell carcinoma: An investigator initiated, proof-of-concept study. J Am Acad Dermatol. 2019;80:564-566.
Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39:1-10.
Drucker AM, Adam GP, Rofeberg V, et al. Treatments of primary basal cell carcinoma of the skin: A systematic review and network meta-analysis. Ann Intern Med. 2018;169:456-466.
Lacouture M. The RegiSONIC disease registry: Preliminary effectiveness and safety in the first 66 newly diagnosed locally advanced basal cell carcinoma (BCC) patients treated with vismodegib. ASCO meeting abstracts. 2015;33:9023.
Lewis K, Peris K, Sekulic A, et al. 428 Interim analysis of Phase 2 results for cemiplimab in patients with metastatic basal cell carcinoma (mBCC) who progressed on or are intolerant to hedgehog inhibitors (HHIs). J Immunother Cancer. 2020;8(suppl 3):A260-A261.
Migden MR, Guminski A, Gutzmer R, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): A multicenter, randomized, double-blind phase 2 trial. Lancet Oncol. 2015;16:716-728.
National Cancer Center Network. NCCN Clinical Practice Guidelines in Oncology. Basal Cell Skin Cancer. Version 1.2022 – November 17, 2021.
Sekulic A, Migden MR, Lewis K, et al. Pivotal ERIVANCE basal cell carcinoma (BCC) study: 12-month update of efficacy and safety of vismodegib in advanced BCC. J Am Acad Dermatol. 2015;72:1021-1026.e8.
Stenger M. Cemiplimab-rwlc for patients with locally advanced basal cell carcinoma. Asco Post. Published April 25, 2021. https://ascopost.com/issues/april-25-2021/cemiplimab-rwlc-for-locally-advanced-and-metastatic-basal-cell-carcinoma/
Zelin E, Zalaudek I, Agozzino M, et al. Neoadjuvant therapy for non-melanoma skin cancer: Updated therapeutic approaches for basal, squamous, and Merkel cell carcinoma. Curr Treat Options Oncol. 2021;22:35.
