Systemic Treatment
Systemic Treatment of Advanced cSCC
Unlike the melanoma and basal cell carcinoma therapeutic landscapes, treatment options for cSCC have not advanced significantly over the last 5-10 years. Current regimens with minimal data and marginal response include cisplatin, interferon alpha, cis-retinoic acid, cetuximab, and gefitinib. Over 15 years ago, Shin and colleagues published their phase II data from 39 patients with advanced cSCC, who received triplet therapy of interferon alpha, cisplatin, and cis-retinoic acid. Overall and complete response rates were 34% and 17%, respectively with one-, two-, and five-year survival rate estimates of 58%, 32%, and 21%, respectively. A small (n=36) phase II study in patients with unresectable cSCC evaluated weekly cetuximab for 7 weeks with a 48-week follow up. The primary study end point – disease control rate at 6 weeks – was 69%. Lastly, a small Phase II study of gefitinib (with individually escalated doses up to 750 mg) in patients with advanced cSCC of the head and neck reported a median progression free survival (PFS) of 1.9 months and median overall survival of 5.1 months. The investigators concluded that gefitinib has clinical activity as monotherapy in SCC of the head and neck. While dose escalation is feasible and may increase skin toxicity, the data do not support increased activity. No large, phase III studies have followed up on these preliminary findings leaving the clinician with very few effective and safe treatment options for advanced and metastatic cSCC. These findings are reflected in the current NCCN guidelines.

Treatment - Cutaneous SCC: Immune Activation as Therapy
Based upon the underlying pathophysiology of cSCC, the high mutational burden of this cancer, and early data, the FDA approved cemiplimab for the treatment of patients with metastatic cSCC or patients with locally advanced cSCC, who are not candidates for surgery.
Early data of cemiplimab showed a response rate of 50% (95% CI, 30-70) and the rate of durable disease control was 65% (95% CI, 44-83). The median observed time to response was 2.3 months and the duration of response exceeded 6 months in 54% of the patients. In metastatic disease, the response rate was 47% (95% CI, 34-61) and the rate of durable disease control was 61% (95% CI, 47-74). A partial response was observed in 24 patients and a complete response (CR) in 4 patients. The median time to response was 1.9 months and the median duration of response was not reached at the time of analysis. The most common adverse events were diarrhea, fatigue, nausea, constipation, and rash.
Ongoing clinical trials are studying the efficacy and tolerability of cemiplimab in this highly underserved malignancy. For example, Rischin and colleagues presented up to three-year data in patients with advanced cSCC from the EMPOWER-CSCC-1 study. Cemiplimab achieved ORR of 46.1%. Overall, the complete response (CR) rate was reported as 16.1% and a median time to CR was 11.2 months with an ORR of 46.1%. A recent study also looked at neoadjuvant use of cemiplimab (treatment given as a the initial step to shrink a tumor before the main treatment, which is usually surgery). Neoadjuvant cemiplimab was associated with a high percentage of pathological complete response (51%) or major response (13%) in those with resectable cSCC.
Another checkpoint inhibitor approved for cSCC is pembrolizumab. The Keynote 629 study investigators examined the efficacy and safety of pembrolizumab in patients with recurrent or metastatic cSCC in an open-label, nonrandomized, global trial. Interim analysis of data after a median follow-up of 11.4 months found a mPFS of 6.9 months and ORR of 34.3%. Treatment-related AEs occurred in 66.7% of patients the most common being pruritus (14.3%), asthenia (13.3%), and fatigue (12.4%). Recent follow up of 14.9 months and 27.2 months, respectively, for both the locally advanced and recurrent/metastatic patients reported ORR of 50% and 35.2%.
Additionally, in the ongoing CARSKIN study, 39 patients with cSCC received first-line pembrolizumab. The primary endpoint was ORR at week 15. The primary cohort’s ORR was 41% (95% CI, 26% to 58%), including 13 partial responses and 3 complete responses. Pembrolizumab-related adverse events affected 71% of the patients, and 4 (7%) were at least grade 3. One death was related to rapid cSCC progression; another resulted from a fatal second aggressive head and neck SCC diagnosed 15 weeks post inclusion.
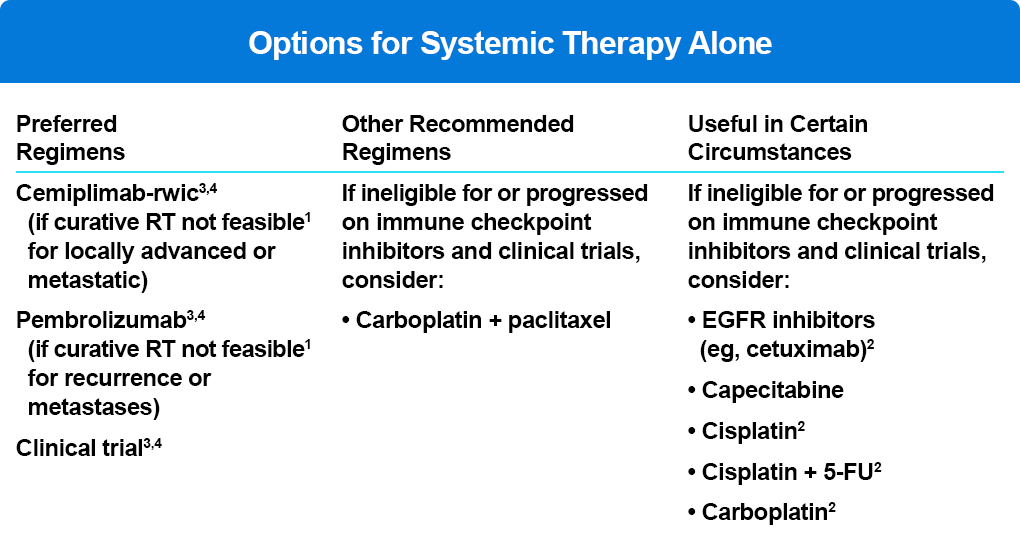
The current NCCN cSCC guidelines reflect the efficacy and tolerability of checkpoint therapy in patients with locally advanced or metastatic disease (Table 2).

References
Borradori L, Sutton B, Shayesteh P, Daniels GA. Rescue therapy with anti-programmed cell death protein 1 inhibitors (PD-1) of advanced cutaneous squamous cell carcinoma and basosquamous carcinoma: Preliminary experience of five cases. Br J Dermatol. 2016;175(6):1382-1386.
Chang AL, Kim J, Luciano R, et al. A case report of unresectable cutaneous squamous cell carcinoma responsive to pembrolizumab, a programmed cell death protein 1 inhibitor. JAMA Dermatol. 2015;152: 106-108.
Dreyfuss I, Kamanth P, Frech F, et al. Squamous cell carcinoma: 2021 updated review of treatment. Dermatol Ther. 2022;35(4):e15308.
Falchook GS, Leidner R, Stankevich E, et al. Responses of metastatic basal cell and cutaneous squamous cell carcinomas to anti-PD1 monoclonal antibody REGN2810. J Immunother Cancer. 2016;4: 70-75.
Grob JJ, Gonzalez R, Basset-Seguin N, et al. Pembrolizumab monotherapy for recurrent or metastatic cutaneous squamous cell carcinoma: A single-arm phase II trial (KEYNOTE-629). J Clin Oncol. 2020;38: 2916-2925.
Gross N, Miller D, Khushalani N, et al. Neoadjuvant Cemiplimab for Stage II to IV Cutaneous Squamous-Cell Carcinoma. N Engl J Med. 2022;387(17):1557-1568.
Hughes BGM, Munoz-Couselo E, Mortier L, et al. Pembrolizumab for locally advanced and recurrent/metastatic cutaneous squamous cell carcionoma (KEYNOTE-629 study): An open-label, nonrandomized, multicenter, phase 2 trial [published online ahead of print, 2021 Jul 19]. Ann Oncol. 2021;S0923-7534(21)02186-4.
Maubec E, Petrow P, Scheer-Senyarich I, et al. Phase II study of cetuximab as first-line single-drug therapy in patients with unresectable squamous cell carcinoma of the skin. J Clin Oncol. 2011;29: 3419-3426. doi:10.1200/JCO.2010.34.1735
Maubec E, Boubaya M, Petrow P, et al. Phase II study of pembrolizumab as first-line, single-drug therapy for patients with unresectable cutaneous squamous cell carcinomas. J Clin Oncol. 2020;38: 3051-3061.
Migden MR, Rischin D, Schmultz CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous cell carcinoma. N Engl J Med. 2018;379: 341-351.
National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology. Squamous cell skin cancer V1.2021. https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf.
Papadopoulos K, Owonikoko T, Johnson M, et al. Cemiplimab (REGN2810): A fully human anti-PD-1 monoclonal antibody for patients with unresectable locally advanced or metastatic cutaneous squamous cell carcinoma (CSCC)—Initial safety and efficacy from expansion cohorts (ECs) of phase I study. J Clin Oncol. 2017;35(15 suppl):9503.
Perez CA, Song H, Raez LE, et al. Phase II study of gefitinib adaptive dose escalation to skin toxicity in recurrent or metastatic squamous cell carcinoma of the head and neck. Oral Oncol. 2012;48: 887-892.
Rischin D, Khushalani NI, Schmults CD, et al. Phase II study of cemiplimab in patients (pts) with advanced cutaneous squamous cell carcinoma (CSCC): Longer follow-up. J Clin Oncol 2020;38:(15 suppl):10018.
Shin DM, Glisson BS, Khuri FR, et al. Phase II and biologic study of interferon alfa, retinoic acid, and cisplatin in advanced squamous skin cancer. J Clin Oncol. 2002;20: 364-370.
