Immunotherapy in BCC
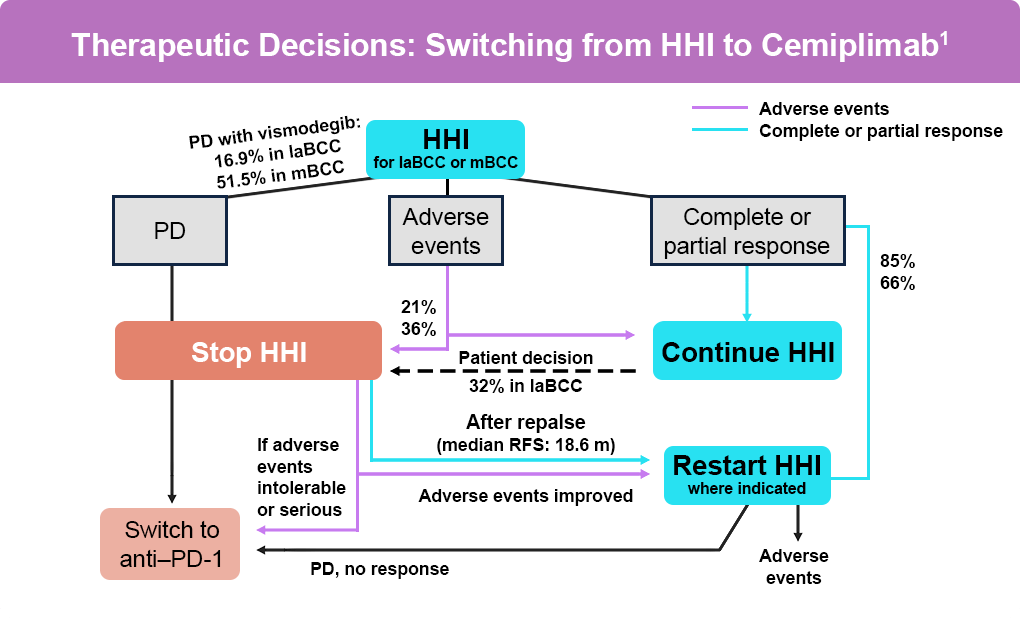
For patients with advanced basal cell carcinoma (BCC), including locally advanced or metastatic BCC not amenable to curative surgery or radiotherapy, hedgehog pathway inhibitors (HHI), such as vismodegib and sonidegib are approved as first-line systemic treatments. However, clinical trials have shown high discontinuation rates for these treatments—88% to 92% with vismodegib and around 92% with sonidegib. The primary factors for discontinuation are efficacy and adverse events. About half of the patients discontinue HHI after 8 to 12 months, often switching to immunotherapy, which is indicated in patients previously treated with an HHI or for whom an HHI is not appropriate (Figure).1,2
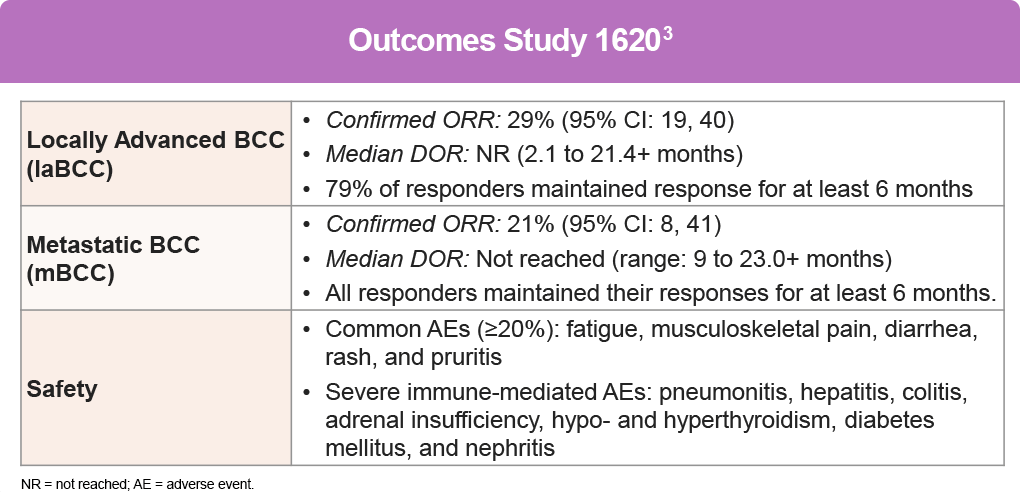
Cemiplimab, an anti–PD-1 agent, was the first immunotherapy to receive full FDA-approval for laBCC and accelerated approval for mBCC as a second-line therapy for patients previously treated with an HHI or for whom an HHI is not appropriate. In studies, cemiplimab has shown efficacy with a safety profile similar to other anti–PD-1 agents in advanced malignancies, including advanced and metastatic BCC.3
A pivotal study for cemiplimab, Study 1620 (NCT03132636) was an open-label, multi-center, non-randomized trial, including participants with advanced BCC (laBCC or mBCC) who had progressed on HHI therapy, had no objective response after 9 months on HHI therapy, or were intolerant of prior HHI therapy. Treatment consisted of cemiplimab 350 mg every 3 weeks for up to 93 weeks until disease progression, unacceptable toxicity, or completion of planned treatment.3,4
One small study aimed to explore the combined effect of Hedgehog pathway inhibitors and immunotherapy, investigating pembrolizumab and vismodegib5:
- Study Details5:
- Participants: 16 patients (9 on pembrolizumab alone, 7 on pembrolizumab/vismodegib)
- Results:
- ORR for all patients: 38%
- ORR for pembrolizumab alone: 44%
- ORR for dual therapy: 29%
- Complete Responses (CRs): One with pembrolizumab monotherapy, two with dual therapy
- Safety: No unexpected toxicity with the combination, but no clear signal of improved efficacy with combination therapy
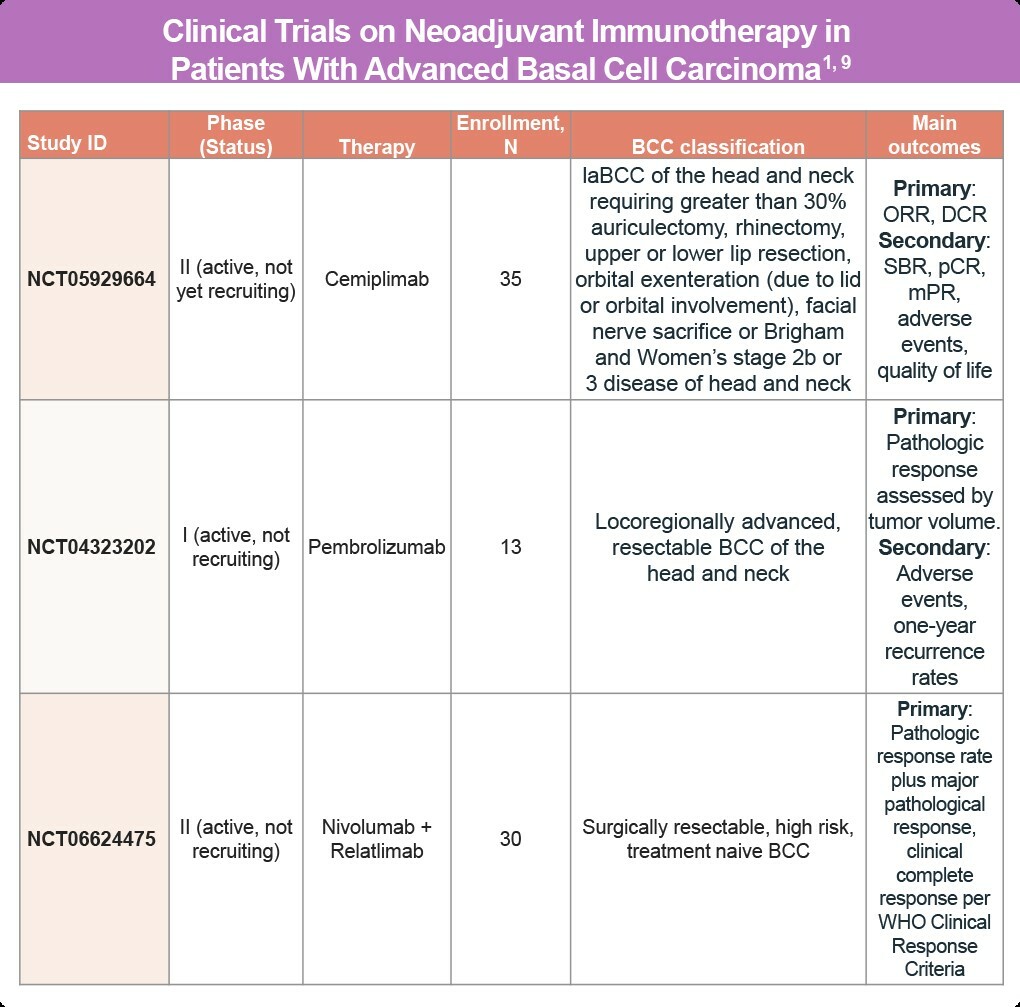
Clinical Trials on Neoadjuvant Immunotherapy for Advanced BCC
Currently, there are no approved neoadjuvant treatments for advanced BCC. However, neoadjuvant anti-PD-1 immunotherapy is being investigated in several clinical trials.1
Ongoing Clinical Trials for Neoadjuvant Immunotherapy:
- Nivolumab: Used as a first-line treatment in two patients with locally advanced BCC (laBCC)6
- Cemiplimab: Investigated for advanced BCC requiring extensive surgical procedures, such as:7
- Greater than 30% auriculectomy
- Rhinectomy
- Upper or lower lip resection
- Orbital exenteration (due to lid or orbital involvement)
- Facial nerve sacrifice
- Brigham and Women’s stage 2b or 3 disease of the head and neck
- Pembrolizumab: Studied in patients with resectable high-risk BCC8
Currently, no guidelines recommend adjuvant anti-PD-1 treatment for resected advanced BCC outside of clinical trials. The use of these therapies remains under investigation to better understand their efficacy and safety in the neoadjuvant setting (Table).1
Anti-PD-1 agents are a therapeutic option for advanced BCC patients not amenable to curative surgery or radiotherapy who have progressed or are intolerant to HHIs. Despite durable responses in some patients, many do not respond or lose their response to treatment. There is currently no validated predictive biomarker to select patients most likely to benefit from anti-PD-1 immunotherapy, underscoring the need for further research into personalized treatment approaches. Identifying and treating patients more likely to respond to anti-PD-1 therapy is a promising area of active research.1
References
- Dessinioti C, Stratigos AJ. Immunotherapy and its Timing in Advanced Basal Cell Carcinoma Treatment. Dermatol Pract Concept. 2023;13:e2023252. doi:10.5826/dpc.1304a252
- National Comprehensive Cancer Network®. NCCN Clinical Practice Guidelines in Oncology. Basal Cell Skin Cancer. Version 1.2026. (https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf).
- Stratigos AJ, Sekulic A, Peris K, et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: An open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021;22:848-857. doi:10.1016/S1470-2045(21)00126-1
- U.S. Food and Drug Administration (FDA). FDA approves cemiplimab-rwlc for locally advanced and metastatic basal cell carcinoma. February 9, 2021.(https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-cemiplimab-rwlc-locally-advanced-and-metastatic-basal-cell-carcinoma).
- Chang ALS, Tran DC, Cannon JGD, et al. Pembrolizumab for advanced basal cell carcinoma: An investigator-initiated, proof-of-concept study. J Am Acad Dermatol. 2019;80:564-566. doi:10.1016/j.jaad.2018.08.017
- Ligtenberg KG, Hu JK, Damsky W, et al. Neoadjuvant anti-programmed cell death 1 therapy for locally advanced basal cell carcinoma in treatment-naive patients: A case series. JAAD Case Rep. 2020;6:628-633. doi:10.1016/j.jdcr.2020.05.010
- Neoadjuvant REGN2810 (Cemiplimab) in Cutaneous Basal Cell Carcinoma of the Head and Neck. ClinicalTrials.gov identifier: NCT05929664. Last updated: July 29, 2025 (https://clinicaltrials.gov/study/NCT05929664).
- A Phase 1B, Single Arm Study of Neoadjuvant-Adjuvant Pembrolizumab in Resectable Advanced Basal Cell Carcinoma of the Head and Neck to Assess for Pathologic Responses in the Tumor Microenvironment. ClinicalTrials.gov identifier: NCT04323202. Last updated: May 15, 2025(https://clinicaltrials.gov/study/NCT04323202).
- Pham JP, Staeger R, Joshua AM, et al. An updated review of immune checkpoint inhibitors in cutaneous oncology: Beyond melanoma. Eur J Cancer. 2025;214:115121. doi:10.1016/j.ejca.2024.11512
All URLs accessed September 12, 2025