Immunotherapy in cSCC
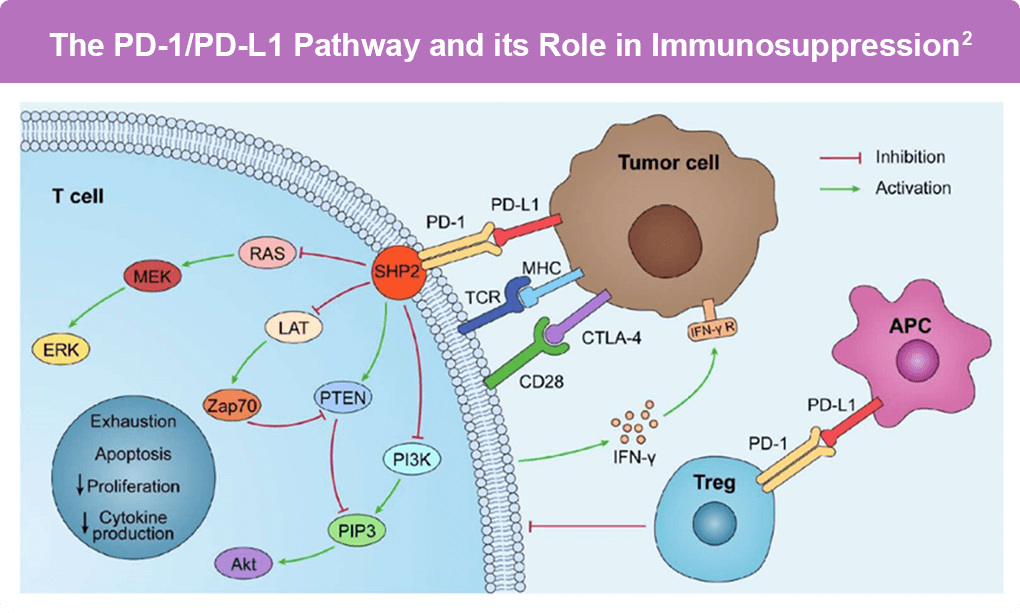
UV radiation is the most significant risk factor for cutaneous squamous cell carcinoma (cSCC), which has one of the highest rates of somatic mutations. This high tumor mutational burden (TMB) leads to numerous neoantigens that can be recognized by the immune system, providing a strong biological rationale for the development of immunotherapies. Indeed, cSCC exhibits a high expression of Programmed Death-Ligand 1 (PD-L1), which interacts with Programmed Death-1 (PD-1) on T cells to inhibit the anti-tumor immune response, allowing cancer cells to evade immune detection (Figure). This mechanism underlies the rationale for using PD-1 inhibitors in cSCC treatment.1,2
Figure. PD-L1 is overexpressed on tumor cells and antigen-presenting cells (APCs) in the tumor microenvironment and interacts with PD-1 on T cells. When PD-L1 binds to PD-1, PD-1 can recruit the tyrosine phosphatase SHP2, leading to the inactivation of CD28 and the T-cell receptor (TCR) function and signaling pathways: PI3K/PIP3/Akt or RAS/MEK/ERK. This results in decreased CD8+ T-cell proliferation, survival, and cytokine production.
Immunotherapy has shown significant efficacy in treating advanced cSCC, particularly when curative radiotherapy or surgery is not feasible for locally advanced, recurrent, or metastatic disease. Two phase II trials, KEYNOTE 629 and CARSKIN, investigated the efficacy of pembrolizumab in cSCC.3 In KEYNOTE-629, among 159 patients with locally advanced or recurrent/metastatic disease treated with pembrolizumab, the objective response rate was 40.9% and the disease control rate was 56.6%. Median duration of response was 52.5 months, with 80.7% of responders maintaining responses for at least 12 months. Median progression-free survival was 8.0 months, and median overall survival reached 29.8 months, with a 36-month OS rate of 47.0%.4 disease-free survival was 89%, and overall survival was 95%.4 In the CARSKIN trial, treatment-naïve patients had an ORR of 42%, with a higher response rate in PD-L1 positive patients compared to PD-L1 negative ones.5
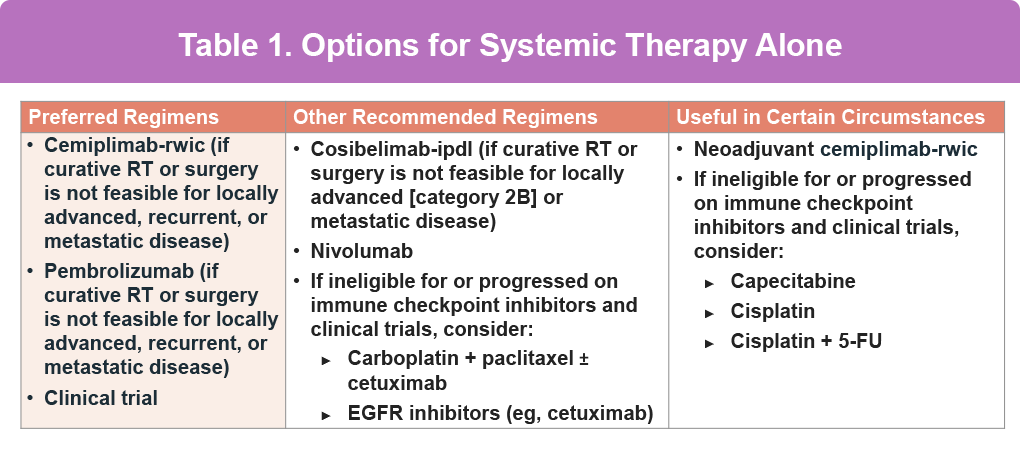
Cemiplimab’s efficacy was demonstrated in the phase II EMPOWER-CSCC 1 study. Patients with metastatic or locally advanced cutaneous squamous cell carcinoma (CSCC) treated with weight-based or fixed-dose cemiplimab achieved an objective response rate of 47.2% among those with metastatic or locally advanced disease receiving weight-based or fixed-dose therapy (n = 193), and 44.8% among patients with newly enrolled metastatic or locally advanced CSCC receiving fixed-dose therapy (n = 165). At 42.5 months, the estimated 12-month duration of response was 88.3%, and median progression-free survival reached 26.0 months; in the newly enrolled cohort, both duration of response and PFS were not yet reached at 8.7 months.6,7 Based on these results the FDA approved cemiplimab for treating metastatic cSCC or locally advanced cSCC ineligible for surgery. Current NCCN guidelines reflect the efficacy and tolerability of systemic therapy for advanced cSCC, including in the neoadjuvant setting (Table 1).8
Despite optimal loco-regional treatment, up to 30% of advanced cSCC patients experience recurrence and up to 5% progress to a stage where curative treatment is not possible. In advanced cases where surgery is borderline feasible, the risk of incomplete resection, disfigurement, and functional morbidity is high. This underscores the rationale for neoadjuvant anti-PD-1 therapy in advanced cSCC.3
Neoadjuvant immunotherapy, using treatment before surgery, is emerging as an exciting approach for patients with resectable but high-risk cSCC. The goal is to shrink tumors, boost immune memory, and potentially reduce the need for extensive surgery.7 In two studies using cemiplimab before surgery, 64–75% of patients had major pathological responses (very little or no remaining tumor).9, 10, 11 Long-term data show no recurrences in those who achieved this level of response.13 The MATISSE trial of neoadjuvant nivolumab ± ipilimumab showed promising results, with some patients avoiding surgery altogether.14
In the C-POST study, cemiplimab was used in the adjuvant setting, or after surgery. Cemiplimab significantly improved 24-month disease-free survival compared with placebo (87% vs 64%), reduced local-regional recurrence (94.6% vs 76.7%) and distant recurrence (94.3% vs 83.8%), with grade ≥3 adverse events in 23.9% vs 14.2%.15
Despite these promising results, two main questions remain. First is whether treatment can be de-escalated in responders to neoadjuvant immunotherapy. The DESQUAMATE trial is exploring this by avoiding surgery in patients with negative restaging after neoadjuvant pembrolizumab. Second, patient selection is crucial, as up to 10% may experience disease progression during neoadjuvant treatment. Gene expression studies have indicated an inflamed tumor microenvironment in responders, but PD-L1 expression and tumor mutational burden are unreliable predictive biomarkers.3
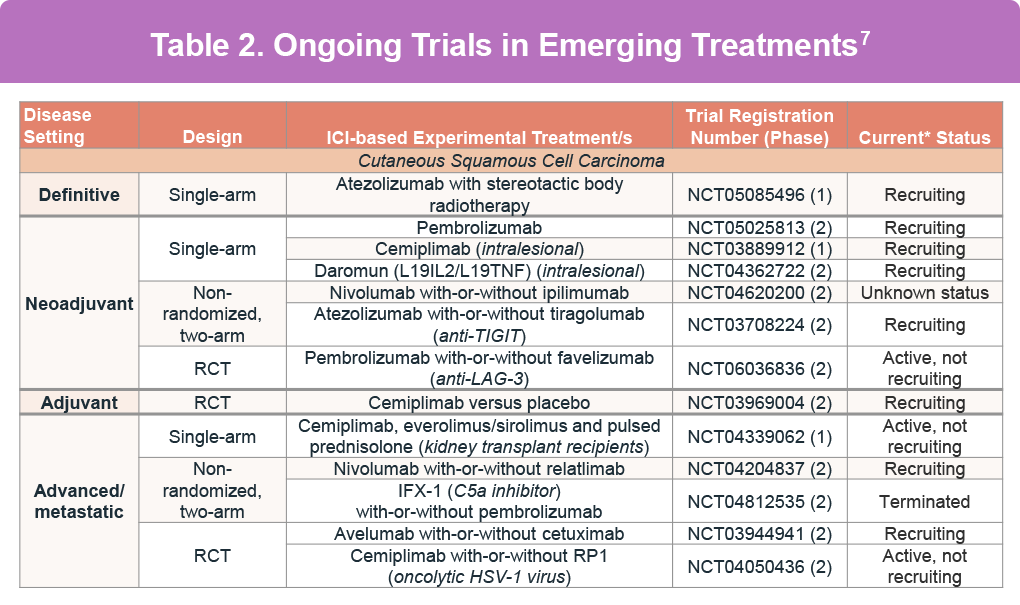
Intralesional treatments, directly administered inside the tumor, offer a selective drug delivery approach, minimizing systemic side effects and preserving function and appearance. This is particularly useful for patients with comorbidities or those who refuse surgery. Several intralesional immunotherapy drugs are currently under clinical trial evaluation (Table 2).12
By understanding the underlying pathophysiology and leveraging recent advancements in immunotherapy, treatment for advanced cSCC continues to improve, offering hope for better patient outcomes.
References
- Boutros A, Cecchi F, Tanda ET, et al. Immunotherapy for the treatment of cutaneous squamous cell carcinoma. Front Oncol. 2021;11:733917. doi:10.3389/fonc.2021.733917
- Zhang Y, Wu J, Zhao C, Zhang S, Zhu J. Recent advancement of PD-L1 detection technologies and clinical applications in the era of precision cancer therapy. J Cancer. 2023;14:850-873. doi:10.7150/jca.81899
- Lorini L, Alberti A, Bossi P. Advanced cutaneous squamous cell carcinoma management in immunotherapy era: Achievements and new challenges. Dermatol Pract Concept. 2023;13:e2023251. doi:10.5826/dpc.1304a251
- Muñoz Couselo E, Hughes BGM, Mortier L, et al. Pembrolizumab (pembro) for locally advanced (LA) or recurrent/metastatic (R/M) cutaneous squamous cell carcinoma (cSCC): Long-term results of the phase 2 KEYNOTE-629 study. J Clin Oncol. 2024;42(16_suppl):9554. doi:10.1200/JCO.2024.42.16_suppl.9554
- Maubec E, Boubaya M, Petrow P, et al. Phase II study of pembrolizumab as first-line, single-drug therapy for patients with unresectable cutaneous squamous cell carcinomas. J Clin Oncol. 2020;38:3051-3061. doi:10.1200/JCO.19.03357
- Hughes BGM, Guminski A, Bowyer S, et al. A phase 2 open-label study of cemiplimab in patients with advanced cutaneous squamous cell carcinoma (EMPOWER-CSCC-1): Final long-term analysis of groups 1, 2, and 3, and primary analysis of fixed-dose treatment group 6. J Am Acad Dermatol. 2025;92(1):68-77. doi:10.1016/j.jaad.2024.06.108
- Pham JP, Staeger R, Joshua AM, et al. An updated review of immune checkpoint inhibitors in cutaneous oncology: Beyond melanoma. Eur J Cancer. 2025;214:115121. doi:10.1016/j.ejca.2024.115121
- National Comprehensive Cancer Network®. NCCN Clinical Practice Guidelines in Oncology. Squamous Cell Skin Cancer. Version 1.2026. (https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf).
- Ferrarotto R, Amit M, Nagarajan P, et al. Pilot phase II trial of neoadjuvant immunotherapy in locoregionally advanced, resectable cutaneous squamous cell carcinoma of the head and neck. Clin Cancer Res. 2021;27:4557-4565. doi:10.1158/1078-0432.CCR-21-0585
- Gross ND, Miller DM, Khushalani NI, et al. Neoadjuvant cemiplimab for stage II to IV cutaneous squamous-cell carcinoma. N Engl J Med. 2022;387:1557-1568. doi:10.1056/NEJMoa2209813
- Ascierto PA, Bossi P, Mandalà M, et al. NEO-CESQ study: Neoadjuvant plus adjuvant treatment with cemiplimab in surgically resectable, high risk stage III/IV (M0) cutaneous squamous cell carcinoma. J Clin Oncol. 2023;41(16_suppl):9576. doi:10.1200/JCO.2023.41.16_suppl.9576
- Baeza-Hernández G, Cañueto J. Intralesional treatments for invasive cutaneous squamous cell carcinoma. Cancers (Basel). 2023;16:158. doi:10.3390/cancers16010158
- Ferrarotto R, Nagarajan P, Maronge JM, et al. Outcomes of Treatment With Neoadjuvant Cemiplimab for Patients With Advanced, Resectable Cutaneous Squamous Cell Carcinoma of the Head and Neck: Secondary Analysis of a Phase 2 Clinical Trial. JAMA Otolaryngol Head Neck Surg. 2023;149(9):847-849.
- Zuur CL, Breukers S, Machuca-Ostos M, et al. Towards organ preservation and cure via 2 infusions of immunotherapy only, in patients normally undergoing extensive and mutilating curative surgery for cutaneous squamous cell carcinoma: An investigator-initiated randomized phase II trial—The MATISSE trial. J Clin Oncol. 2023;41(16_suppl):95. doi:10.1200/JCO.2023.41.16_suppl.95
- Rischin D, Porceddu S, Day F, et al. Adjuvant Cemiplimab or Placebo in High-Risk Cutaneous Squamous-Cell Carcinoma. N Engl J Med. Published online May 31, 2025. doi:10.1056/NEJMoa2502449
All URLs accessed September 16, 2025